Practice Essentials
Cryotherapy is the ablation of tissue through local induction of extremely cold temperatures. Cryotherapy can be used both for primary treatment of prostate cancer and for salvage treatment of disease refractory to radiation therapy. [1]
Relative contraindications to cryotherapy include previous transurethral resection of the prostate (TURP) with a large tissue defect, as well as significant symptoms of urinary tract obstruction. A history of abdominoperineal resection for rectal cancer, rectal stenosis, or other major rectal pathology is also a relative contraindication.
A preprocedure prostate-specific antigen (PSA) test is important for assessing risk and establishing a baseline from which the PSA level can be tracked after treatment. Other preprocedure laboratory studies include the following:
-
Urine culture
-
Complete blood cell (CBC) count with platelet count
-
Coagulation tests (ie, prothrombin time [PT] and activated partial thromboplastin time [aPTT])
The use of a cryotherapy system involves placement of cryoprobes under ultrasonographic guidance bilaterally in the anteromedial, posterolateral, and posteromedial regions of the gland, to the proximal extent of the prostatic capsule. See the image below.
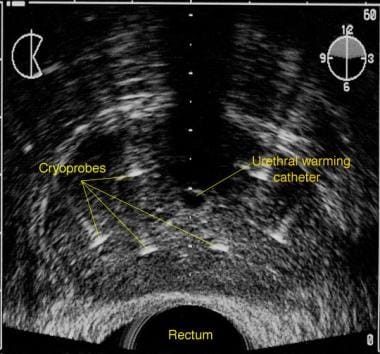 Transrectal sonogram of the prostate illustrating placement of the cryoprobes and urethral-warming catheter.
Transrectal sonogram of the prostate illustrating placement of the cryoprobes and urethral-warming catheter.
Complications of cryotherapy for prostate cancer include the following:
-
Impotence
-
Incontinence
-
Tissue sloughing
-
Pelvic and rectal pain
-
Penile numbness
-
Rectourethral fistula
-
Urethral stricture
-
Hydronephrosis
-
Small-bowel obstruction
Background
Cryotherapy—the ablation of tissue through local induction of extremely cold temperatures—has its earliest antecedent in 19th-century London, where Arnott applied ice-salt mixtures to cancers of the breast and cervix. [2] The 1966 advent of probes cooled by liquid nitrogen in closed circulation marks the beginning of modern cryotherapy. [3]
One of the first applications of this new technology was the transurethral cryoablation of benign prostatic hyperplastic tissue, [4] followed shortly thereafter by the treatment of prostate cancer via an open perineal approach. [5] The transperineal approach was introduced in 1974, initially using a single digitally guided cryoprobe that was repositioned as needed during the procedure. [6]
Early series of cryotherapy achieved effective tissue ablation, and complications were considered to be less severe than those of radical surgery at the time. The major impediment to early acceptance of the modality, however, was the inability to achieve accurate monitoring of cryoprobe placement and ice-ball formation.
Subsequent advances, which have reinvigorated investigation into the use of cryotherapy for prostate cancer, have included the use of real-time transrectal ultrasonography (TRUS) to monitor probe placement and freezing, [7] the simultaneous use of multiple cryoprobes, and the standard use of urethral warming catheters. [8]
A particularly significant development was the introduction of cryotherapy probes that use argon gas rather than liquid nitrogen. Argon rapidly cools the probe tip to –187°C (–304.6°F) and can be rapidly exchanged with helium at 67°C (152.6°F) for an active thawing phase, producing a faster response to operator input and significantly speeding 2-cycle treatment. [9]
Moreover, argon-based probes have a much smaller diameter than nitrogen-based probes. Thus, they permit direct, sharp transperineal insertion, rendering tract dilation unnecessary and facilitating more conformal cryosurgery by allowing placement of more probes. [10]
Since the mid-1990s, cryotherapy has seldom been used in community urologic practice, despite the initiation of Medicare reimbursement for the procedure in 1999. According to polls conducted by the American Urological Association (AUA), the percentage of urologists performing cryosurgery from 1997-2001 remained constant at 2%, though the average annual number of procedures performed by each urologist increased from 4 to 24. [11]
In contrast, the percentage of urologists performing brachytherapy over the same period rose from 16% to 51%, and the annual number of procedures performed by each urologist rose from 15 to 16.5. [11]
Nevertheless, ongoing technical advances and accumulating results from academic centers suggest that cryotherapy may be poised to capture an increased role as an alternative to other, more standard approaches to management of localized prostate cancer.
Outcomes have now been reported as late as 7 years after treatment and seem to compare favorably with those reported in contemporary series of patients who receive radiation therapy, particularly with respect to late failure rates [12] and among higher-risk patients. [13]
It must be acknowledged, however, that even the largest cryotherapy studies have been retrospective examinations of largely single-institution experiences. Moreover, they have used disparate definitions of clinical risk, biochemical failure, continence, and potency; these definitions all must be standardized for cryotherapy.
For fair comparisons to be made with other modalities, prospective studies, ideally randomized, must be conducted; such studies should use consistent definitions, even across treatment modalities, and must control for clinical risk parameters. Given the relative paucity of alternatives for patients who experience biochemical progression after radiotherapy, cryosurgery may also prove a good alternative for those whose tumors appear to remain localized despite progression.
For patient education information, see the Prostate Health Center and the Cancer and Tumors Center, as well as Prostate Cancer.
Indications
Cryotherapy can be used both for primary treatment of prostate cancer and for salvage treatment of disease refractory to radiation therapy.
Primary treatment
A research summary by the Agency for Healthcare Research and Quality (AHRQ) concluded that because of the lack of relevant randomized controlled trials, it is not known whether cryotherapy is more or less effective than other therapies in the treatment of localized prostate cancer. [14]
The AUA published a Best Practice Statement on the use of cryosurgery for the treatment of localized prostate cancer. [15] The statement concluded that level II-2/3 evidence exists to support offering cryotherapy to men with clinically organ-confined prostate cancer with a negative metastatic evaluation finding. In high-risk men, including those with clinical stage T3 disease, the data are sparser; multimodal therapy may be necessary.
As with any other treatment for prostate cancer, appropriate patient selection is critical, and preprocedure tumor characteristics are strong indicators of outcome. The best outcomes can be expected in patients with low-risk tumor features (ie, serum prostate-specific antigen [PSA] level ≤10 ng/mL, diagnostic biopsy Gleason score ≤6, and clinical stage T1c or T2a).
Patients with higher-grade, more extensive, or more advanced disease are at higher risk for local extension, metastatic spread, or both. Cryoablation has, however, been used for local disease control in patients with known metastatic disease who are receiving systemic therapy but require palliative maneuvers for local symptoms. [16]
A study by Tay et al indicated that in patients with high-grade, clinically localized prostate cancer, primary cryotherapy seems in the community setting to be a safe and effective treatment in the short term. The study included men with a biopsy Gleason score of 8 or above and localized (cT1-2) prostate cancer, as well as a serum PSA level of 50 ng/mL or less. The investigators found the estimated rate of biochemical progression–free survival at 2 and 5 years to be 77.2% and 59.1%, respectively. Patient-reported rates of complete continence and potency at 12-month follow-up were 90.5% and 17%, respectively. According to multivariate analysis, biochemical progression was significantly associated with a Gleason score of 9 or 10 and a lowest posttreatment PSA reading (PSA nadir) of 0.4 ng/mL or above. [17]
Salvage treatment
The AUA Best Practice Statement concluded that level II-3 evidence supports the consideration of cryotherapy in men in whom radiation therapy has failed, particularly those with biopsy-proven local persistence or recurrence, clinically localized disease, and a PSA level lower than 10 ng/mL. [15]
Few local treatment alternatives are available for patients who do not achieve a low PSA nadir or who experience a rising PSA level after radiotherapy. Additional brachytherapy [18] and radical prostatectomy [19] are options; however, most patients in this position undergo systemic androgen deprivation therapy, which may control the cancer for several years but does not offer the possibility of definitive cure.
Cryosurgery has been established as a viable alternative for patients in whom radiotherapy has failed. [20] Tumor cells resistant to radiotherapy, androgen withdrawal, and chemotherapy may remain vulnerable to the physical trauma of freezing and thawing.
Candidates for such salvage treatment should be carefully selected. In particular, if the goal is cure, the treating physician must be reasonably confident that the failure of radiation therapy is truly attributable to persistent or recurrent local disease rather than to occult metastatic disease.
To this end, inclusion criteria for reported series of salvage cryotherapy have generally included imaging tests such as nuclear scintigraphy to rule out metastases to the bones and pelvic cross-sectional imaging with computed tomography (CT) or magnetic resonance imaging (MRI) to rule out metastases to the pelvic lymph nodes. However, the sensitivity of these tests, particularly those for lymph node involvement, is less than 50%, [21] and the likelihood of positive test results despite a low PSA level is quite low. [22]
Some investigators have confirmed the presence of viable, treatable local disease by means of prostate biopsy. [23] In patients with high-risk features, such as a preirradiation PSA level higher than 20 ng/mL, a Gleason score of 8-10, or a rapidly rising PSA level after radiation therapy, a pelvic lymphadenectomy (performed either via laparoscopy or via a minilaparotomy) may be considered.
Independent of prostate cancer, patients should have a life expectancy of at least several years, and they should understand the increased risks of adverse effects in the context of salvage therapy. Most reported procedures have been performed in patients whose conditions have proved refractory to external-beam radiotherapy, but success has also been reported in patients with disease refractory to brachytherapy. [23]
Contraindications
Relative contraindications to cryotherapy include previous transurethral resection of the prostate (TURP) with a large tissue defect, as well as significant symptoms of urinary tract obstruction. A history of abdominoperineal resection for rectal cancer, rectal stenosis, or other major rectal pathology is also a relative contraindication.
In most contemporary series, cryotherapy is associated with higher rates of impotence than other local treatment alternatives are. Accordingly, patients for whom preservation of erectile function is a high priority are probably less than ideal candidates.
Cryotherapy for larger prostates can be problematic because of the difficulty in achieving a uniformly cold temperature throughout the gland. Even with multiple probes, complete ablation of glands larger than 50 cm3 is difficult, and multiple probe insertions and prolonged freezing times may be required. In these cases, the prostate may be cytoreduced with neoadjuvant hormonal ablation before cryoablation. [24]
Because cryotherapy is not used to stage or treat pelvic lymph nodes, patients at high risk for lymph node metastasis may not be ideal candidates for cryotherapy. The AUA Best Practice Statement suggests that prior or concurrent lymph node dissection may be warranted for such patients. [15] These patients may also be more appropriately managed with a different primary treatment modality.
The most sensitive and readily available test for detecting metastases to the bones is nuclear scintigraphy (bone scan). Scintigraphy is recommended as a staging test in patients who present with a PSA level that exceeds 10 ng/mL, a Gleason score higher than 7, or indications of bone pain.
The goal of cross-sectional imaging in the setting of prostate cancer is to reveal local extension or metastases to the lymph nodes. Unfortunately, the sensitivity of available imaging modalities remains low.
CT scans reveal only 25-45% of lymph node metastases and 55-75% of cases of bulky local extension. CT scanning is therefore recommended only in patients at high risk for advanced disease (eg, those with a PSA level >20 ng/mL, a Gleason score of 8-10, or clinical stage T3-T4 disease). [25]
The sensitivity of MRI for lymph node metastasis detection is not superior to that of CT scanning. [21] The use of endorectal coils and adjunctive magnetic resonance spectroscopy may eventually improve the utility of this test for local staging, but it is currently not recommended for routine clinical practice.
Technical Considerations
Best practices
Technical measures
The following technical measures were explicitly recommended by the AUA Best Practice Statement panel [15] :
-
Use of rapid freezing for better tissue destruction
-
Use of thermocouples for temperature monitoring
-
Freezing to –40°C
-
Use of passive (slow) thawing
-
Use of a double freeze-thaw cycle
Reduction of prostate size
A large prostate volume (>50 cm3) can reduce the technical feasibility of complete cryoablation. For example, neoadjuvant androgen ablation with a 3-month depot injection of a luteinizing hormone–releasing hormone agonist usually reduces the prostate to 60-70% of its original size. Androgen ablation may also reduce the tumor burden in patients with stage T3 disease (ie, gross extracapsular extension or seminal vesicle involvement).
In the setting of interstitial radiotherapy (brachytherapy), neoadjuvant androgen ablation has indeed been shown to effectively reduce prostate size; although it does not affect oncologic outcomes, it does diminish quality-of-life outcomes, particularly in terms of potency rates. [26, 27]
Neoadjuvant androgen ablation has not been prospectively evaluated in the context of cryotherapy; however, in a subset analysis of retrospective series, it was not shown to improve outcomes. [28] Indeed, in a large analysis, subjects receiving neoadjuvant therapy had worse biochemical outcomes than those receiving cryotherapy alone, although patients in the neoadjuvant group also had more aggressive tumor characteristics on average. [13]
Regional lymphadenectomy
Patients at high risk for lymph node metastasis who are contemplating cryosurgery but have negative findings on cross-sectional imaging studies may undergo regional lymphadenectomy to assess for metastasis. Lymphadenectomy may be performed laparoscopically or via a minilaparotomy with low morbidity.
Identification of lymph node metastases is a relative contraindication for aggressive local therapy for prostate cancer. Even if resected lymph nodes prove to be free of disease, significant risk factors for lymph node metastases, such as a PSA level higher than 20 ng/mL or a Gleason score of 8-10, predict failure after any local treatment.
Subtotal prostate cryotherapy
There is growing interest in focal therapy for prostate cancer, which uses targeted radiation or energy-based ablation techniques to treat a focus of cancer while sparing the rest of the prostate and surrounding structures. The goal is better quality-of-life preservation among men with low-risk, presumably localized tumors.
The primary difficulty with focal therapy is that prostate cancer is frequently multifocal, and its distribution cannot be reliably identified by any currently available imaging modality. Furthermore, even extended-template biopsies may undersample the prostate, resulting in understaging, undergrading, or underappreciation of multifocality.
A few small series of subjects undergoing focal, unilateral, or otherwise subtotal cryotherapy have been reported, but this approach is generally considered experimental. The AUA Best Practice Statement concluded, on the basis of level III evidence, that cases of subtotal prostate cryosurgery should be described and collected prospectively in a database and studied more rigorously before a treatment recommendation can be made. [15]
A subsequent report from the national Cryo On-Line Database (COLD) Registry found that the oncologic efficacy of focal therapy appeared to be similar to that of whole-gland cryoablation. [29] The report also found that the impact of focal cryoablation on urinary, sexual, and bowel function appeared to be less than that of radical therapies, although it did not preserve sexual function as well as might have been expected.
Procedural planning
Relevant anatomy
The prostate gland rests in the pelvis on the urogenital diaphragm. It lies inferior to the bladder, anterior to the rectum (from which it is separated by Denonvilliers aponeurosis [fascia]), and posterior to the retropubic space of Retzius. It is bounded bilaterally by the levator ani musculature. The prostate surrounds the prostatic urethra.
The prostate receives its blood supply from the inferior vesical and middle rectal branches of the internal iliac arteries and drains via the Santorini dorsal venous plexus. Innervation is via the pelvic plexus arising from the T10-T12 and S2-S4 nerve roots. The neurovascular bundles run inferolaterally to the prostate and are critical determinants of penile erectile function.
The prostate is divided into zones that describe the ductal drainage systems. The posterior peripheral zone accounts for 70% of the prostate volume and is the location of 60-70% of prostate cancers. The transition zone accounts for only 5% of normal prostate volume but is the site of all benign prostatic hyperplasia (BPH) and is therefore frequently enlarged. From 10-20% of prostate cancers are located in the transition zone. The central zone accounts for 25% of prostate volume and is involved in 5-10% of prostate cancers.
Outcomes
Cryotherapy for local control
Among patients undergoing repeat biopsy 3-24 months after treatment with a standard 5-probe cryotherapy system, 7.7-25% have been found to have residual malignancy of the glands, [30, 31, 32] and 42-71% have been found to have focal areas of viable benign epithelium. [33, 34, 32] Numerous disease- and treatment-related factors have been shown to predict rates of local control.
In one series, for example, the likelihood of positive biopsy findings was 9% in subjects with clinical stage T1 or T2 disease, compared with 21% in those with T3 disease. [35] Persistent or recurrent cancer is more likely among tumors located in the prostatic apex or the seminal vesicles than those located in the midgland or the base. [33]
A pooled analysis stratified patients into the following risk groups [13] :
-
Low risk - PSA level of 10 ng/mL or lower, Gleason score of 6 or less, and clinical stage T1 or T2a disease
-
Intermediate risk - PSA level higher than 10 ng/mL, Gleason score of 7 or more, or clinical stage T2b disease or higher
-
High risk - The presence of 2 or 3 of these adverse risk factors
The distribution of patients among low-, intermediate-, and high-risk groups was 25%, 34%, and 41%, respectively. The positive biopsy rate in the series was 18% overall: 12% among low- and intermediate-risk patients and 24% among high-risk patients.
The use of 2 freeze-thaw cycles rather than 1 reduced the positive biopsy rate from 64% to 11% in a series of primary cryotherapy patients [33] and from 29% to 9% among a group treated with salvage cryotherapy for radiation failure. [16] Other technical advances have also produced improvements.
One series reported a reduction of the positive biopsy rate from 83% to 10% as a result of introducing the use of thermosensors during treatment. [36] Another series reported a positive biopsy rate of only 2.5% with the use of 6-8 cryotherapy probes rather than the conventional 5 probes. [37]
Biochemical failure (primary therapy)
Defining biochemical recurrence after local prostate cancer treatment is controversial; by one count, 152 different definitions were used in surgical and radiation studies published between 1991 and 2004—53 in prostatectomy series and 99 in radiation series. [38] This variation in definition creates great difficulty in comparing outcomes across treatment modalities and is no less of a problem in the case of cryotherapy.
PSA thresholds of 0.4, 0.5, and 1 ng/mL have all been used, as have both the original American Society for Therapeutic Radiology and Oncology (ASTRO) definition of 3 consecutive rises after a nadir and the updated Phoenix definition of nadir plus 2 ng/mL. [15] The ASTRO and Phoenix definitions tend to delay identification of treatment failure relative to threshold definitions, thereby artificially improving success rates in survival analyses. [39, 40]
No specific definition was endorsed by the AUA Best Practice Statement. [13] However, tissue response to cryotherapy should be more rapid than the response to radiation therapy, and thus, a nadir should be reached more rapidly—within 3 months of treatment in most cases following a sharp posttreatment rise. Therefore, threshold definitions may be more appropriate than the ASTRO or Phoenix definitions.
Cryotherapy does not ablate every gland in the prostate at the microscopic level. Consequently, the target nadir has not been established with certainty, although the nadir achieved initially clearly correlates with the eventual disease progression. A report of cryotherapy experiences in a community setting indicated that 84% of the patients reached a PSA nadir below 0.4 ng/mL, though the follow-up period was quite short. [41]
Biochemical failure, defined as a rise in PSA level of 0.2 ng/mL after a nadir of less than 0.5 ng/mL, was reported to be lowest in subjects whose PSA nadirs were below 0.1 ng/mL. [33] Similarly, positive biopsy rates were 1.5%, 10%, and 55% in subjects with nadirs that were less than 0.1, 0.1-0.5, and more than 0.5 ng/mL, respectively.
In a pooled analysis with a median follow-up of 24 months, actuarial 5-year biochemical disease-free survival (bDFS) rates were 60%, 45%, and 36% for low-, intermediate-, and high-risk patients, respectively, when failure was defined on the basis of a PSA threshold of 0.5 ng/mL. When a threshold of 1 ng/mL was used, bDFS rates were 76%, 71%, and 45%, respectively. [13]
Prepelica et al reported a series of 65 men with high-risk prostate cancer, defined as a PSA level of 10 ng/mL or higher or a Gleason score of 8 or higher, and found an 83.3% bDFS rate according to the ASTRO definition at median 35-month follow-up. In this series, 50% of patients achieved a nadir PSA of less than 4 ng/mL, and 35% achieved a nadir of less than 1 ng/mL.
Morbidity was low in this study, with 2 patients reporting incontinence, 2 patients reporting rectal pain, and 2 patients reporting urinary retention. Roughly two thirds of the patients in this cohort had received neoadjuvant hormonal therapy, the survival impact of which is still unclear in association with cryotherapy. [42]
One of the larger series of patients to date, with the longest follow-up, included 590 subjects followed for a mean of 5.4 years. [12] The reported 7-year bDFS rates were stratified by the same risk definitions used by Long et al [13] but used several different definitions.
When an absolute PSA threshold of 0.5 ng/mL was used to define failure (as in many surgical series), the bDFS rates were 61%, 68%, and 61% for low-, intermediate-, and high-risk subjects. [12] When the ASTRO definition of failure (ie, 3 successive rises in PSA level) was used, the bDFS rates were 92%, 89%, and 89%.
In all, 13% of subjects had positive biopsy findings; of these patients, 32 underwent repeat cryoablation, with 7-year bDFS rates comparable to those who had primary cryoablation only: 68% with the 0.5 ng/mL threshold and 91% with the ASTRO definition. Relatively few late failures occurred beyond 24-36 months. [12]
Jones et al published the largest series of patients undergoing cryotherapy as primary treatment, all of whom were included in the industry-sponsored COLD registry. [43] The series included 1198 men managed by 27 physicians. The median pretreatment PSA level was 6.8 ng/mL (mean, 9.6 ± 8.6 ng/mL), and various Gleason scores were represented (median, 7); 49.5% of these men received hormone therapy before cryoablation. The mean follow-up period was 24.4 ± 25.9 months.
Five-year actuarial biochemical recurrence-free survival rates were 77.1% ± 2.1% by the ASTRO definition and 72.9% ± 2.1% by the Phoenix definition. Risk-stratified outcomes are listed in the Table below. One caveat is that this report somewhat misuses the Phoenix definition, which is intended to predict outcomes only at a point 2 years short of the median follow-up; thus, to report 5-year outcomes, 7 years of follow-up should be available. [44] Therefore, outcomes may be expected to worsen somewhat with further follow-up.
Table. Risk-Stratified Outcomes of Studies of Cryotherapy for Prostate Cancer (Open Table in a new window)
Study |
No. of Patients |
Residual Cancer, % |
Median Follow-up Period |
bDFS Criterion |
bDFS, % |
Onik et al [7] |
23 |
17 |
3 mo |
. . . |
. . . |
Miller et al [45] |
62 |
21 |
3 mo |
. . . |
. . . |
Bahn et al [30] |
130 |
8 |
. . . |
. . . |
. . . |
Coogan et al [46] |
87 |
17 |
1 y |
≤0.2 ng/mL |
33 |
Wieder et al [47] |
61 |
13 |
3 mo |
< 0.5 ng/mL |
57 |
Bales et al [34] |
23 |
14 |
1 y |
< 0.3 ng/mL |
14 |
Shinohara et al [33] |
102 |
23 |
3 mo |
< 0.1 ng/mL |
48 |
Wake et al [31] |
63 |
25 |
3 mo |
< 0.1 ng/mL |
25 |
Cohen et al [48] |
383 |
18 |
2 y |
< 0.4 ng/mL |
55 |
Pisters et al [16] |
150 |
18 |
. . . |
< 0.2 ng/mL |
46 |
Lee et al [37] |
81 |
3 |
. . . |
. . . |
. . . |
Gould [49] |
27 |
. . . |
6 mo |
< 0.2 ng/mL |
96 |
Long et al [13] |
975 |
18 |
24 mo |
< 0.5 ng/mL |
60 (low risk), 45 (intermediate risk), 36 (high risk) |
Bahn et al [12] |
590 |
13 |
5.4 y |
< 0.5 ng/mL |
61 (low risk), 68 (intermediate risk), 61 (high risk) |
Han et al [50] |
106 |
. . . |
1 y |
< 0.4 ng/mL |
75 (78 low risk, 71 high risk) |
Prepelica et al [42] |
65 (all high-risk) |
. . . |
35 mo |
ASTRO < 1 ng/mL |
83 35 |
Cresswell et al [51] |
51 |
. . . |
9 mo |
< 0.5 ng/mL |
79 |
Jones et al [43] |
1198 |
14.5/38.4 |
2 y |
ASTRO/Phoenix |
85 (low risk), 73 (intermediate risk), 75 (high risk) |
bDFS = biochemical disease-free survival. |
|||||
Salvage cryotherapy
Patients who experience disease progression after radiation therapy have few options for potentially curative therapy. Cryotherapy has been offered to such patients if they have no evidence of metastatic disease and their progression is thought to be restricted to persistent or recurrent local cancer.
Contemporary series have demonstrated promising results for this treatment approach. In a series of 150 subjects, an approach that used 2 freeze-thaw cycles yielded a negative biopsy rate of 93% and a biochemical failure-free survival rate of 66%, [16] although these results came at the price of higher complication rates. [52] Subjects with preoperative PSA levels higher than 10 ng/mL or biopsy Gleason scores of more than 8 were most likely to experience disease recurrence. [16]
A biochemical failure-free survival rate of 66% at 12 months was reported in a series of 43 salvage patients, with lower complication rates [53] ; additionally, a PSA nadir higher than 0.1 ng/mL after treatment predicted eventual recurrence. [53] With the use of an argon-based cryosurgery system to treat 38 patients with biochemical recurrence after radiation, PSA nadirs lower than 0.1 ng/mL were reported in 81.5% and bDFS rates of 86% and 74% were reported at 1- and 2-year follow-up, respectively. [54]
In a large series that also used an argon-based system, 118 subjects with recurrent disease after radiation therapy underwent cryoablation, including 5 who had received permanent interstitial implants. [23] Negative biopsy findings were reported in 94% of these patients; the 7 who had persistent disease underwent second ablation procedures.
PSA nadirs below 0.5 ng/mL occurred in 97% of subjects; at a median of 18.6 months of follow-up, 34% remained below this level (68% had PSA levels < 4 ng/mL). Ten patients had developed metastatic disease. [23] As in the 1997 study by Pisters et al, [16] preprocedure PSA levels higher than 10 ng/mL, Gleason scores greater than 8, and stage T3-T4 disease predicted biochemical failure.
Pisters et al reported outcomes for salvage cryotherapy among 279 men from the COLD registry, the largest salvage series to date. [55] These patients had significant recurrent/persistent disease, with a mean precryotherapy PSA level of 7.6 ± 8.2 ng/mL and a mean Gleason score of 7.5, and 51% of them received hormone therapy before cryotherapy, for a mean of 13 months.
After a follow-up period of 21.6 ± 24.9 months, 17% of the patients had a PSA level lower than 0.2 ng/mL at 5 years of follow-up. The actuarial biochemical recurrence-free survival rate at 5 years was 58.9% ± 5.7% by the ASTRO definition and 54.5% ± 4.9% by the Phoenix definition. Forty-six patients underwent biopsy after cryotherapy; results were positive in 32.6%. [55]
It should be noted that the follow-up in this study, as in the COLD registry study of primary therapy, [43] was insufficient to allow proper reporting of 5-year outcomes according to these definitions. [44]
Multiple prostate cancer studies over the past few years have generated increased awareness of the importance of PSA kinetics, which are assessed with measurements such as PSA velocity (PSAV) and PSA doubling time (PSADT) at various stages of prostate cancer management. To date, only one study has examined PSA kinetics in relation to cryotherapy.
Spiess et al analyzed 49 patients undergoing salvage cryotherapy for failure after radiation therapy for predictors of biochemical outcomes, and they found that both precryotherapy PSA levels higher than 10 ng/mL and a PSADT of 16 months or less predicted biochemical recurrence after cryotherapy. [56] The median presalvage PSA level was 5.9 ng/mL, and 51% of the patients had a preirradiation Gleason grade of 8 or greater.
Studies of salvage radiotherapy after surgery have consistently found that the PSA level at the time of radiotherapy predicts outcome, [57] as have studies of salvage prostatectomy after failure of radiation therapy. [58] Thus, earlier use of cryotherapy at lower postirradiation PSA levels may improve outcomes, but this issue has not yet been studied in depth.
Choi et al conducted a study of the use of intensity-modulated radiation therapy in the treatment of adenocarcinoma of the prostate after cryotherapy failure. [59] Their study cohort consisted of 9 patients who were treated from 2008 to 2010. The median follow-up period was 31 months (range, 15–40 months). The mean preradiotherapy prostate-specific antigen level was 4.3 ng/mL (range, 1.07–15.6 ng/mL). The median elapsed time between cryotherapy and radiation therapy was 20.5 months (range, 8.5–56.5 months). Biochemical control was achieved in 7 patients. Two patients developed distant metastases shortly after completion of radiotherapy. No patients experienced toxicities of grade 3 or higher. The investigators concluded that high-dose intensity-modulated radiation therapy following failure of cryotherapy is well tolerated without severe morbidity and that through its use, a significant number of patients can become biochemically free of disease after failure of initial ofcryotherapy. [59]
Longer-term salvage data are also becoming available. Bahn et al reported 7-year outcomes for 59 patients treated with cryotherapy after failed radiation therapy. The bDFS rate was 59% with a PSA threshold of 0.5 ng/mL and 69% with a threshold of 1 ng/mL. Notably, there were no local recurrences upon repeat biopsy; all failures were presumably due to distant progression. [60]
Future directions for improving outcomes
Ongoing developments in technology and clinical algorithms may be expected to facilitate improvements in cryotherapy outcomes with respect to cancer control and quality of life. Varying the intensity and extent of cryoablation should allow patients and physicians to contemplate tradeoffs between quality of life and cancer control certainty.
Gould has described a technique of total cryosurgery, in which no urethral warming catheter is used and the urethra is intentionally ablated along with the entire prostate gland. [49] With this technique, he achieved PSA nadirs of less than 0.2 ng/mL at 6 months in 96% of his patients, compared with 49% for standard cryotherapy and 73% for a contemporary series of patients who underwent radical perineal prostatectomy. These results came at the cost of obstruction necessitating TURP in most patients and significant incontinence in 18.5%. [49]
A novel simulator system, which promises to facilitate training in cryosurgical techniques, has been introduced. [61]
On another frontier, Rukstalis et al have suggested that prostate parenchyma-sparing cryosurgery may improve outcomes in terms of continence and potency. Despite the multifocal nature of prostate cancer, in an analysis of 112 radical prostatectomy specimens, they found that by assuming the largest tumor would be the one detected by biopsy, restricting treatment to 9 of 12 prostate zones, and thereby sparing the contralateral neurovascular bundle, they could accomplish treatment with a 21% risk of significant (ie, >0.5 mL) residual disease. [62]
Onik et al have tested a similar approach in a small series of 9 patients treated with focal, unilateral nerve-sparing cryotherapy. [63] At a mean follow-up of 36 months, all 9 patients had stable PSA levels, 6 patients had biopsy specimens with negative findings, and 7 were potent. [63]
In a pilot program at the University of California at San Francisco, 8 patients with localized prostate cancer likewise have been treated with unilateral cryotherapy. All are disease-free, either on the basis of PSA criteria or on the basis of negative biopsy results. None of the 8 patients developed a urethral fistula or significant tissue sloughing. Significantly, although only 2 were potent before treatment, both reported no change in their erections after the limited cryosurgical treatment.
Onik et al reported on a larger series (55 men) who underwent treatment only of the area of the prostate known to harbor cancer, finding that at a mean follow-up of 3.6 years, 95% had stable PSA levels (ASTRO definition), and 86% of potent patients retained their erectile function. [64] Although such approaches should still be considered experimental, they hold significant promise as the indications and approaches to focal therapy continue to evolve.
Innovative combination therapy may also play a role in the future. Clarke et al reported in vitro data suggesting that the combination of 5-fluorouracil (5-FU) and cryotherapy produces efficient prostate cancer cell death at temperatures as high as –15°C, via a mixture of necrosis, Bax-mediated apoptosis, and freeze rupture. [65, 66, 67] In theory, this chemocryotherapy approach could improve outcomes by achieving more uniform tumor death and decrease adverse effects by reducing the ice-ball size required to achieve a higher target temperature.
Other biologic response modifiers, such as antifreeze proteins, may also improve the efficiency of the freezing process, [68] as may improved imaging modalities.
-
Diagram illustrating dimensions of a typical ice ball as seen end-on (left) and from the side (right).
-
Example of third-generation prostate cryotherapy setup, illustrating urethral warming catheter, 2 percutaneous temperature probes, and 3 cryotherapy probes. (This case was a salvage case of focal treatment for an ultrasonographically visible lesion, so only 3 cryoprobes were required.)
-
Transrectal sonogram of the prostate illustrating placement of the cryoprobes and urethral-warming catheter.
-
Transrectal sonogram of the prostate during cryoablation. The leading edge of the ice ball, growing posteriorly, is echodense and casts a dark acoustic shadow anteriorly.
-
Transrectal sonogram illustrating the ice ball now extending posteriorly to the muscularis propria of the rectum. All prostate tissue is now included within the margin of the ice ball.






