Practice Essentials
Hypercalcemia can result when too much calcium enters the extracellular fluid or when there is insufficient calcium excretion from the kidneys. Approximately 90% of cases of hypercalcemia are caused by hyperparathyroidism or malignancy. [1, 2, 3]
Signs and symptoms
The severity of symptoms is related not only to the absolute calcium level but also to how fast the rise in serum calcium occurred. Mild prolonged hypercalcemia may produce mild or no symptoms, or recurring problems such as kidney stones. Sudden-onset and severe hypercalcemia may cause dramatic symptoms, usually including confusion and lethargy, possibly leading quickly to death. Serum calcium levels greater than approximately 15 mg/dL usually are considered to be a medical emergency and must be treated aggressively.
Hypercalcemia affects nearly every organ system in the body, but it particularly affects the central nervous system (CNS) and the kidneys. CNS effects include the following:
-
Lethargy
-
Muscle weakness
-
Bone pain
-
Confusion
-
Stupor
-
Coma
Renal effects include the following:
-
Polyuria
-
Nephrogenic diabetes insipidus
-
Nocturia
-
Dehydration
-
Renal stones
-
Distal renal tubular acidosis
-
Acute kidney injury
-
Chronic kidney disease
Gastrointestinal effects include the following:
-
Constipation
-
Nausea
-
Anorexia
-
Pancreatitis
-
Gastric ulcer
Cardiac effects include
-
syncope
-
Arrhythmias
-
Hypertension
-
Bradycardia
-
Shortening of QT interval
Calcium has a positive inotropic effect. Hypercalcemia also causes hypertension, presumably from renal dysfunction and direct vasoconstriction.
Diagnosis
Hypercalcemia may be classified based on total serum and ionized calcium levels, as follows:
-
Mild: Total Ca 10.5-11.9 mg/dL (2.5-3 mmol/L) or Ionized Ca 5.6-8 mg/dL (1.4-2 mmol/L)
-
Moderate: Total Ca 12-13.9 mg/dL (3-3.5 mmol/L) or Ionized Ca 8-10 mg/dL (2-2.5 mmol/L)
-
Hypercalcemic crisis: Total Ca 14-16 mg/dL (3.5-4 mmol/L) or Ionized Ca 10-12 mg/dL (2.5-3 mmol/L)
Hypercalcemia from malignancy usually is rapidly progressive; thus, rapidly rising calcium levels should increase suspicion of malignancy. Hypercalcemia from hyperparathyroidism is usually mild, asymptomatic, and sustained for years. Immunoreactive parathyroid hormone (PTH) and ionized calcium should be simultaneously measured.
Other causes of hypercalcemia usually can be distinguished or at least considered on the basis of history and physical examination findings. Measurement of serum phosphate, alkaline phosphatase, serum chloride, serum bicarbonate, urinary calcium, and thyroid function may be useful in some cases.
Management
Treatment of hypercalcemia includes the following:
-
Volume repletion with isotonic sodium chloride solution
-
Loop diuretics
-
Bisphosphonates
-
Denosumab
-
Peritoneal dialysis or hemodialysis
-
Surgical correction of hyperparathyroidism
See also:
For patient education information, see Hypercalcemia (Elevated Calcium Levels).
Pathophysiology
Calcium plays an important role in intracellular and extracellular metabolism controlling such processes as nerve conduction, muscle contraction, coagulation, electrolyte and enzyme regulation, and hormone release. Calcium metabolism, in turn, is tightly regulated by a series of hormones that affect not only the entry of calcium into the extracellular space from bone and the GI tract but also control its excretion from the kidneys.
Calcium hemostasis
Ninety-eight percent of body calcium is found in the skeleton; this is closely related to the extracellular concentration of calcium. Intracellular calcium is less than extracellular calcium by a factor of 100,000. Intracellular processes, including the activity of many enzymes, cell division, and exocytosis, are controlled by intracellular calcium. The primary mediator of the intracellular effects of calcium is the calcium-binding regulatory protein, calmodulin.
Plasma calcium is maintained despite its large movements across the gut, bone, kidney, and cells. Changes in calcium ions usually are accompanied by changes in total calcium in the ECF. In plasma, calcium exists in 3 different forms: (1) 50% as ionized or the biologically active form, (2) 45% bound to plasma proteins (mainly albumin), and (3) 5% complexed to phosphate and citrate. Because the proportion of bound calcium varies little within individuals, in the absence of severe acidosis or alkalosis, the amount of albumin is the major factor determining the amount of calcium that is bound.
Very little evidence suggests that intracellular stores of calcium contribute in any way to plasma calcium homeostasis. An exception is in the parathyroid gland, in which the intracellular concentration increases in response to changes in extracellular concentration, which in turn alters the rate of parathyroid hormone (PTH) secretion. Any decrease in extracellular calcium ion concentration leads to an increase in PTH secretion. PTH increases distal renal tubular reabsorption of calcium within minutes and stimulates osteoclast activity, with release of calcium from the skeleton within 1-2 hours. More prolonged PTH elevation stimulates 1alpha-hydroxylase activity in the proximal tubular cells, which leads to 1,25-dihydroxyvitamin D (1,25(OH)2 D3) production. All these mechanisms help to maintain the serum calcium level within normal limits.
A normal serum calcium level is 8-10 mg/dL (2-2.5 mmol/L) with some inter-laboratory variation in the reference range, and hypercalcemia is defined as a serum calcium level greater than 10.5 mg/dL (>2.5 mmol/L). Hypercalcemia may be classified based on total serum and ionized calcium levels, as follows:
-
Mild: Total Ca 10.5-11.9 mg/dL (2.5-3 mmol/L) or ionized Ca 5.6-8 mg/dL (1.4-2 mmol/L)
-
Moderate: Total Ca 12-13.9 mg/dL (3-3.5 mmol/L) or ionized Ca 8-10 mg/dL (2-2.5 mmol/L)
-
Hypercalcemic crisis: Total Ca 14-16 mg/dL (3.5-4 mmol/L) or ionized Ca 10-12 mg/dL (2.5-3 mmol/L)
Only 1-2% of total body calcium is in the exchangeable form in circulation, and the rest forms part of the skeleton. Only one half of the exchangeable calcium is in the active ionized form, with the remainder bound to albumin, globulin, and other inorganic molecules. Protein binding of calcium is influenced by pH with metabolic acidosis leading to increased ionized calcium from reduced protein binding, and alkalosis leading to reduced ionized calcium from increased protein binding. Because calcium binds to albumin and only the unbound (free or ionized) calcium is biologically active, the serum level must be adjusted for abnormal albumin levels.
For every 1-g/dL drop in serum albumin below 4 g/dL, measured serum calcium decreases by 0.8 mg/dL. Therefore, to correct for an albumin level of less than 4 g/dL, one should add 0.8 to the measured value of calcium for each 1-g/dL decrease in albumin. Without this correction, an abnormally high serum calcium level may appear to be normal.
A patient with a serum calcium level of 10.3 mg/dL but an albumin level of 3 g/dL appears to have a normal serum calcium level. However, when corrected for the low albumin, the real serum calcium value is 11.1 mg/dL (10.3 + 0.8), a more obviously abnormal level. Alternatively, serum free (ionized) calcium levels can be directly measured, negating the need for correction for albumin. Corrected calcium can be calculated using the following formula:
Corrected Ca = (4 - plasma albumin in g/dL) × 0.8 + serum calcium
Mild cases of hypercalcemia can be asymptomatic and are more often diagnosed incidentally from routine blood tests. Because calcium metabolism normally is tightly controlled by the body, even mild persistent elevations above normal signal disease and should be investigated.
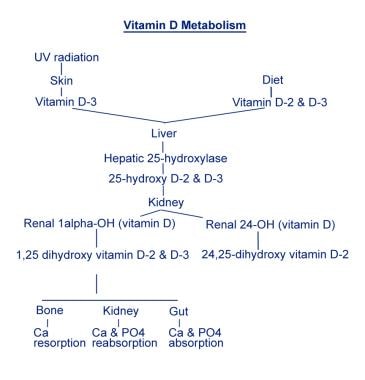
Calcium is controlled by 2 mechanisms: (1) controlling or major regulatory hormones and (2) influencing hormones. Controlling or major regulatory hormones include PTH, calcitonin, and vitamin D. The image below reviews vitamin D metabolism. In the kidney, vitamin D and PTH stimulate the activity of the epithelial calcium channel and the calcium-binding protein (ie, calbindin) to increase active transcellular calcium absorption in the distal convoluted tubule. [4] Influencing hormones include thyroid hormones, growth hormone, and adrenal and gonadal steroids.
Role of the calcium-sensing receptor
The calcium-sensing receptor (CaSR) is a G protein–coupled receptor, which allows the parathyroid chief cells, the thyroidal C cells, and the ascending limb of the loop of Henle (renal tubular epithelial cells) to respond to changes in the extracellular calcium concentration. The ability of the CaSR to sense the serum Ca++ is essential for the appropriate regulation of PTH secretion by the parathyroid glands and for the regulation of passive paracellular calcium absorption in the loop of Henle. Calcitonin secretion and renal tubular calcium reabsorption also are directly regulated by the action of Ca++ on the calcium receptor. [5]
The CaSR gene is located on band 3q13-q21 and encodes a 1078 amino acid protein. CaSR is expressed in many tissues. Three uncommon human disorders are due to abnormalities of the CaSR gene: familial benign hypocalciuric hypercalcemia, neonatal severe hyperparathyroidism, and autosomal dominant hypocalcemia with hypercalciuria. [6, 7]
Etiology
Approximately 90% of cases of hypercalcemia are caused by hyperparathyroidism or malignancy. About 20-30% of patients with cancer have hypercalcemia during the course of the disease, and its occurrence may signify an unfavorable prognosis. Of the cases that result from malignancy, approximately 80% are due to the effects of parathyroid hormone–related peptide (PTHrP), while the other 20% are due to bony metastases. Hypercalcemia secondary to malignancy may be classified into the following four types, based on the mechanism involved:
-
Humoral hypercalcemia of malignancy (HHCM) from an increased secretion of PTHrP - Most common form, accounting for up to 80% of cases
-
Osteolytic hypercalcemia from osteoclastic activity and bone resorption surrounding the tumor tissue - The second most common mechanism, accounting for about 20% of cases
-
Secretion of active vitamin D by some lymphomas
-
Ectopic parathyroid hormone (PTH) secretion - Very rare
The remaining 10% of cases of hypercalcemia are caused by many different conditions, including vitamin D–related problems; disorders associated with rapid bone turnover; and, in rare cases, familial disorders. Treatment with recombinant human PTH for postmenopausal osteoporosis is also a cause. [8]
Causes of hypercalcemia that are related to malignancy (most commonly, lung cancer, breast cancer, and myeloma) include the following:
-
Solid tumor metastases
-
Solid tumors with humoral effects
-
Hematologic malignancies
Causes of hypercalcemia that are related to the parathyroid include the following:
-
Primary hyperparathyroidism - Solitary adenoma, generalized hyperplasia, multiple endocrine neoplasia type 1 or type 2A
-
Lithium-related release of PTH
-
Familial high PTH levels
-
Neonatal severe hyperparathyroidism
-
Kidney transplantation [9]
Causes related to vitamin D include the following:
-
Granulomatous disease (especially sarcoidosis [1] )
Causes related to high bone turnover include the following:
-
Hyperthyroidism
-
Immobilization (especially in Paget disease)
-
Thiazide diuretic use [12]
-
Vitamin A intoxication
-
Kidney failure (milk-alkali syndrome)
Other causes related to particular mechanisms are as follows:
-
Increased intestinal calcium absorption
-
Idiopathic infantile hypercalcemia (Williams syndrome)
-
Granulomatous disorders (eg, sarcoidosis) [1]
-
Decreased renal calcium excretion
-
Familial hypocalciuric hypercalcemia [13]
-
Increased bone resorption
-
Mutations of the calcium-sensing receptor
-
Familial benign hypocalciuric hypercalcemia
-
Hypophosphatasia
-
Subcutaneous fat necrosis
-
Blue diaper syndrome
-
Dietary phosphate deficiency
-
Tuberculosis [14]
Epidemiology
United States
Hypercalcemia is relatively common and often is mild but of long duration. The incidence of hyperparathyroidism alone is approximately 1-2 cases per 1000 adults. Mild cases are often not diagnosed. A review of cancer-related hypercalcemia found that rates varied by tumor type, being highest in multiple myeloma (7.5–10.2%) and lowest in prostate cancer (1.4–2.1%). [15]
International
Screenings of large groups of patients have found prevalence rates as high as 39 cases per 1000 persons in Scandinavia. Similar screenings in South Africa showed a prevalence of 8 cases per 1000 persons. These higher incidences may reflect underdiagnosis in the United States rather than a true difference in prevalence.
Mortality/Morbidity
Morbidity and mortality from hypercalcemia depend entirely on the cause.
Hypercalcemia from hyperparathyroidism tends to be mild and prolonged. Morbidity is related to the resultant bone disease. Because this condition is underdiagnosed so often, actual morbidity is unknown. Mild hypercalcemia rarely, if ever, leads directly to death.
Some studies suggest that up to 20% of patients who present to the ED with hypercalcemia are ultimately diagnosed with hyperparathyroidism. Royer et al performed a retrospective review from 2012 to 2013 of patients with hypercalcemia in the ED, and a definitive diagnosis of hyperparathyroidism was identified in 3.5% (6 of 168). According to the authors, 24% (41 of 168) identified with mild hypercalcemia were discharged from the ED with no definitive follow-up plan, and although mild hypercalcemia found during ED workup rarely requires immediate medical treatment, many of those patients may have hyperparathyroidism amenable to surgical correction. The authors therefore suggested that an appropriate mechanism for outpatient hypercalcemia workup should be integrated into the patient's ED discharge plan. [16]
Hypercalcemia caused by a neoplasm tends to be much more serious. The mechanism of hypercalcemia in malignancy can be from the ectopic production of a PTH-like factor, PTH-related protein (PTHrP), or osteolytic metastases. Cancers that produce PTHrP include breast cancer, lung cancer, prostate cancer, and multiple myeloma. [17]
PTHrP increases the expression of receptor activator of nuclear factor kappa B ligand (RANKL) in bone. RANKL in turn contributes to the development of hypercalcemia by binding to receptor activator of nuclear factor kappa B (RANK) on the surface of osteoclast precursors, leading to bone osteolysis.
Hypercalcemia is often the immediate cause of death in patients with ectopic PTHrP production. These patients rarely survive more than a few weeks or months. Osteolytic metastases tend to cause morbidity and mortality from nerve compression and other orthopedic complications. These patients may live longer but still have a poor prognosis, especially if their serum calcium levels are very high.
Morbidity and mortality associated with hypercalcemia from other causes are directly related to the underlying cause and tend to be less serious. In these patients, hypercalcemia is a reflection of their disease state and morbidity and mortality depend on control of the underlying disease.
Sex- and Age-related Variances
Some studies show a higher incidence in men compared to women, but this difference tends to diminish with increasing age. One study found the highest incidence to be in women aged 60-63 years.
Hypercalcemia from nearly all causes increases with advancing age. That is especially true of hypercalcemia from the two most common causes, malignancy and hyperparathyroidism. However, hypercalcemia may occur in persons of any age.
Prognosis
Cancer-related hypercalcemia most often occurs in later-stage malignancies and it predicts a poor prognosis for patients with it. [18]
In a retrospective matched-control study of 180 patients with lymphoma, Vallet et al found that hypercalcemia was independently associated with poor progression-free and overal survival. The study included 62 patients with hypercalcemia, with 118 comparable patients serving as controls. [19]
In a study of 90 patients with advanced head and neck squamous cell carcinoma (HNSCC), Alsirafy et al compared outcomes for those patients in the cohort who had hypercalcemia (46 patients) with those of patients who did not. The authors found that inpatients with hypercalcemia had a higher rate of palliative care referrals. Moreover, during the final 3 months of patient follow-up, a greater percentage of individuals with hypercalcemia paid more than 1 visit to the emergency room and a larger proportion of hypercalcemic patients were hospitalized for at least 14 days. [20]
The authors also determined that among the study's patients who were referred for palliative care, the median postreferral survival time for those with hypercalcemia was 43 days, while that for nonhypercalcemic patients was 128 days. Alsirafy et al concluded that if hypercalcemia in patients with HNSCC is detected and managed early, this may help to prevent hypercalcemia-associated symptoms and to reduce hospitalization time. [20]
-
Investigations flowchart.
-
Vitamin D metabolism.